Amyloid Precursor Protein (APP) or Amyloid-beta precursor protein acts as a cell-surface membrane receptor for kinesin I, responsible for the axonal transfer of beta-secretase and presenilin 1. APP is essential for neuronal growth, neurite adhesion, and the process of axonogenesis.
The APP protein is a 100-140kDa transmembrane glycoprotein that exists in various isoforms that result from alternative splicing. The proteolytic cleavage of APP through secretases causes the formation beta-amyloid, which is the principal part of plaques that are senile. You can also know more about a beta-Amyloid/APP Antibody online.
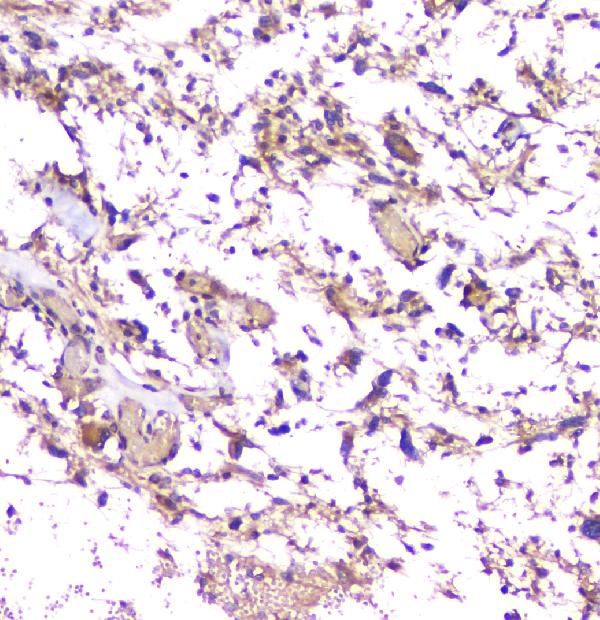
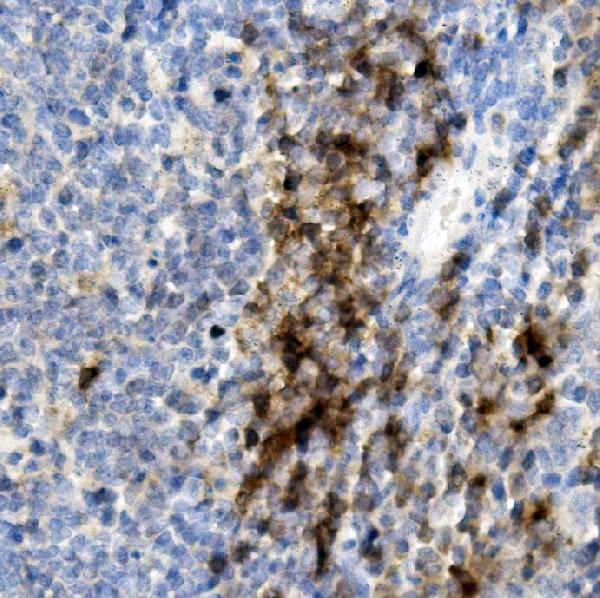
Senile plaques are one the most significant histopathologic manifestations associated with Alzheimer’s. A lack of regulation and processing of the APP is also a factor for Down’s Syndrome, the early onset of familial Alzheimer’s disease and cerebral hemorrhage. This monoclonal antibody recognizes the human mouse and the rat the APP (Amyloid Precursor Protein).
The APP protein is expressed in high levels within the central nervous system. It comprises three distinct isoforms that result from alternative splicing. APP plays an important role in the formation of synaptic connections and repair and repair, neuronal anterograde transport, iron export and hormonal regulation.
The secreted APP (sAPP) could be neuroprotective against neurotoxic injury as well as oxidative stress and excitotoxicity. APP is part of a family that includes at most two homologs, amyloid precursors like proteins 2 and 1 (APLP1 and the APLP2). The resemblances in APP and APP and APP, particularly APLP2 suggests that APLP may be able to share and take over the functions of APP.